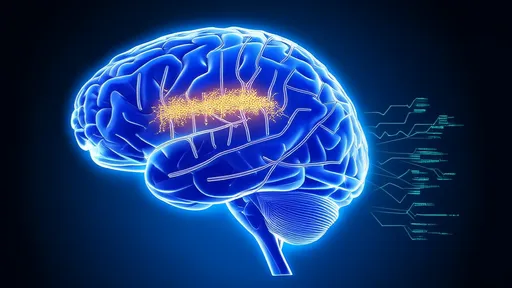
In a groundbreaking development for neurotechnology, researchers have unveiled a new class of neural interfaces using liquid metal electrodes that promise to revolutionize brain-machine communication. These adaptive electrodes, designed to mimic the mechanical properties of brain tissue, address long-standing challenges in neural recording and stimulation by seamlessly integrating with the dynamic biological environment of the human brain.
The conventional rigid electrodes used in neurological applications have always presented a fundamental mismatch with soft brain tissue. This mechanical disparity leads to inflammatory responses, signal degradation over time, and potential tissue damage. The emergence of gallium-based liquid metal alloys as conductive materials has opened unprecedented possibilities for creating neural interfaces that move and flex with the living brain.
What makes these liquid metal electrodes extraordinary is their unique combination of conductivity and fluidity. Unlike solid conductors, these materials maintain excellent electrical performance while possessing the ability to deform and adapt to the brain's subtle movements. When encapsulated within specially designed biocompatible polymers, the liquid metal forms conductive pathways that remain stable even under continuous mechanical stress.
Recent studies published in Nature Neuroscience demonstrate that these interfaces can maintain high-fidelity signal recording for months without the signal degradation typically seen with traditional electrodes. The liquid metal's self-healing properties prevent the formation of micro-fractures that plague conventional rigid electronics when subjected to the brain's constant pulsations.
The clinical implications of this technology are profound. For patients with neurological disorders requiring long-term monitoring or deep brain stimulation, such as Parkinson's disease or epilepsy, these adaptive interfaces could provide more reliable treatment with reduced side effects. The electrodes' gentle mechanical interaction with brain tissue significantly decreases the foreign body response that often leads to scar tissue formation around implants.
Beyond medical applications, the technology shows promise for advancing fundamental neuroscience research. The improved signal stability and longevity allow for more accurate studies of neural networks over extended periods. Researchers can now observe how neural circuits reorganize during learning or recovery from injury without the confounding factors introduced by traditional electrode arrays.
Manufacturing these liquid metal neural interfaces involves innovative microfabrication techniques. Scientists have developed methods to pattern the liquid metal within elastomeric substrates, creating stretchable circuits that maintain conductivity even when stretched to twice their original length. This flexibility is crucial for accommodating the brain's natural movements during everyday activities.
One particularly exciting advancement is the development of injectable liquid metal electrodes that can conform to deep brain structures without requiring invasive surgical placement. These minimally invasive approaches could make neural interface procedures safer and more accessible while reducing recovery times for patients.
As the technology matures, researchers are working to integrate additional functionalities into the liquid metal platforms. Some teams are experimenting with incorporating drug delivery systems that can release neuroprotective compounds directly to the surrounding tissue. Others are developing hybrid systems that combine the liquid metal electrodes with optical components for optogenetic stimulation.
The road ahead still contains challenges that need addressing before widespread clinical adoption. Long-term biocompatibility studies are ongoing to ensure the materials remain stable in the body for decades. Researchers are also working to scale up production while maintaining the precision required for neural interfaces. Nevertheless, the progress made thus far suggests that liquid metal electrodes represent a significant leap forward in our ability to interface with the human brain.
This innovation comes at a critical time as the field of brain-computer interfaces gains momentum. Major technology companies and research institutions are racing to develop systems that can translate neural activity into digital commands. The advent of liquid metal electrodes provides these efforts with a more reliable and biocompatible platform for chronic neural recording and stimulation.
Looking forward, we can anticipate seeing these adaptive interfaces being tested in human clinical trials within the next few years. As the technology proves itself in real-world applications, it may fundamentally change how we treat neurological conditions and interact with technology using our thoughts. The marriage of materials science and neuroscience through liquid metal electrodes marks an exciting new chapter in medical technology that blurs the line between biology and machine.
By /Aug 14, 2025
By /Aug 14, 2025
By /Aug 14, 2025

By /Aug 14, 2025
By /Aug 14, 2025
By /Aug 14, 2025

By /Aug 14, 2025
By /Aug 14, 2025
By /Aug 14, 2025
By /Aug 14, 2025

By /Aug 14, 2025

By /Aug 14, 2025

By /Aug 14, 2025

By /Aug 14, 2025

By /Aug 14, 2025
By /Aug 14, 2025

By /Aug 14, 2025
By /Aug 14, 2025

By /Aug 14, 2025

By /Aug 14, 2025